Depuy Hip Replacement Recall 2017

The company has several lines of knee systems and parts designed for different patient needs for revision surgeries for partial replacements and for total replacements.
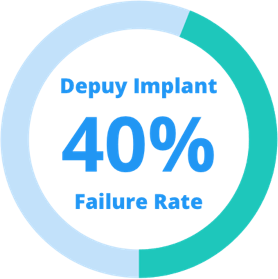
Depuy hip replacement recall 2017. Joint registry revealed that metal devices had more problems than older devices and they were. Depuy knee and hip failures are similar and lead to class action lawsuits 2 8 2019. Depuy is a large and well known manufacturer and marketer of joint replacement implants that operates worldwide. Depuy a leading maker of knee and hip replacement systems has faced more recalls for knee components and systems than any other manufacturer of artificial knees.
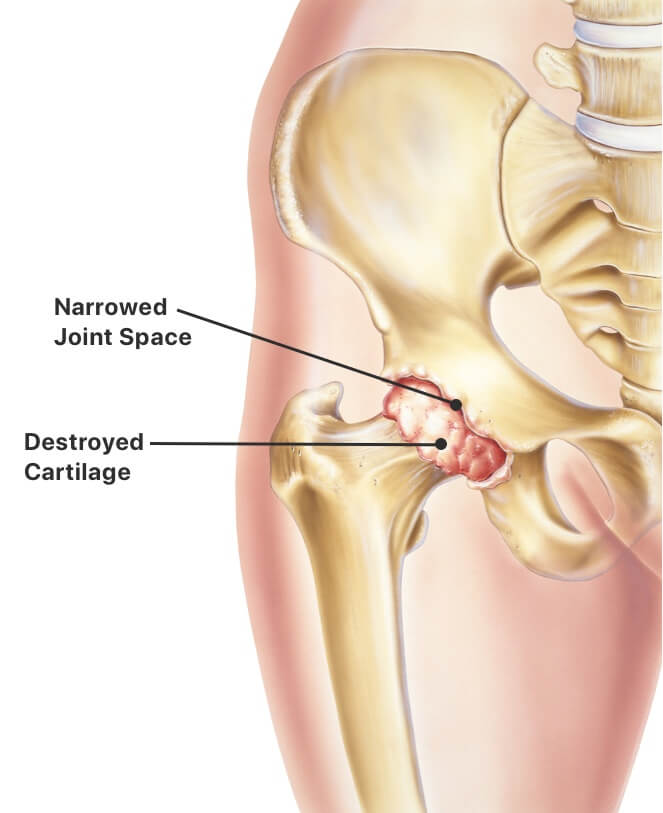
Johnson johnson s history of knee replacement recalls 7 31 2017. Today three prominent hip implant cases are proceeding in federal court with patients from across the country accusing stryker depuy synthes and smith nephew of selling unreasonably dangerous medical devices. The first depuy hip replacement recall lawsuit case in nevada settled out of court in august 2012. In some cases problems arise from the surgery but in many other instances side effects from the hip device have led to additional health issues.
Depuy hip recall lawsuit news information october 16 2017 depuy hip implant lawsuits spread to uk. The depuy pinnacle hip claims saw major plaintiff success in 2016 a momentum that has continued into 2017. Kransky received 338 236 12 in economic and 8 million in non economic damages but the jurors. While the last ten years saw an unbelievable explosion in hip replacement litigation 2017 has proved no exception.
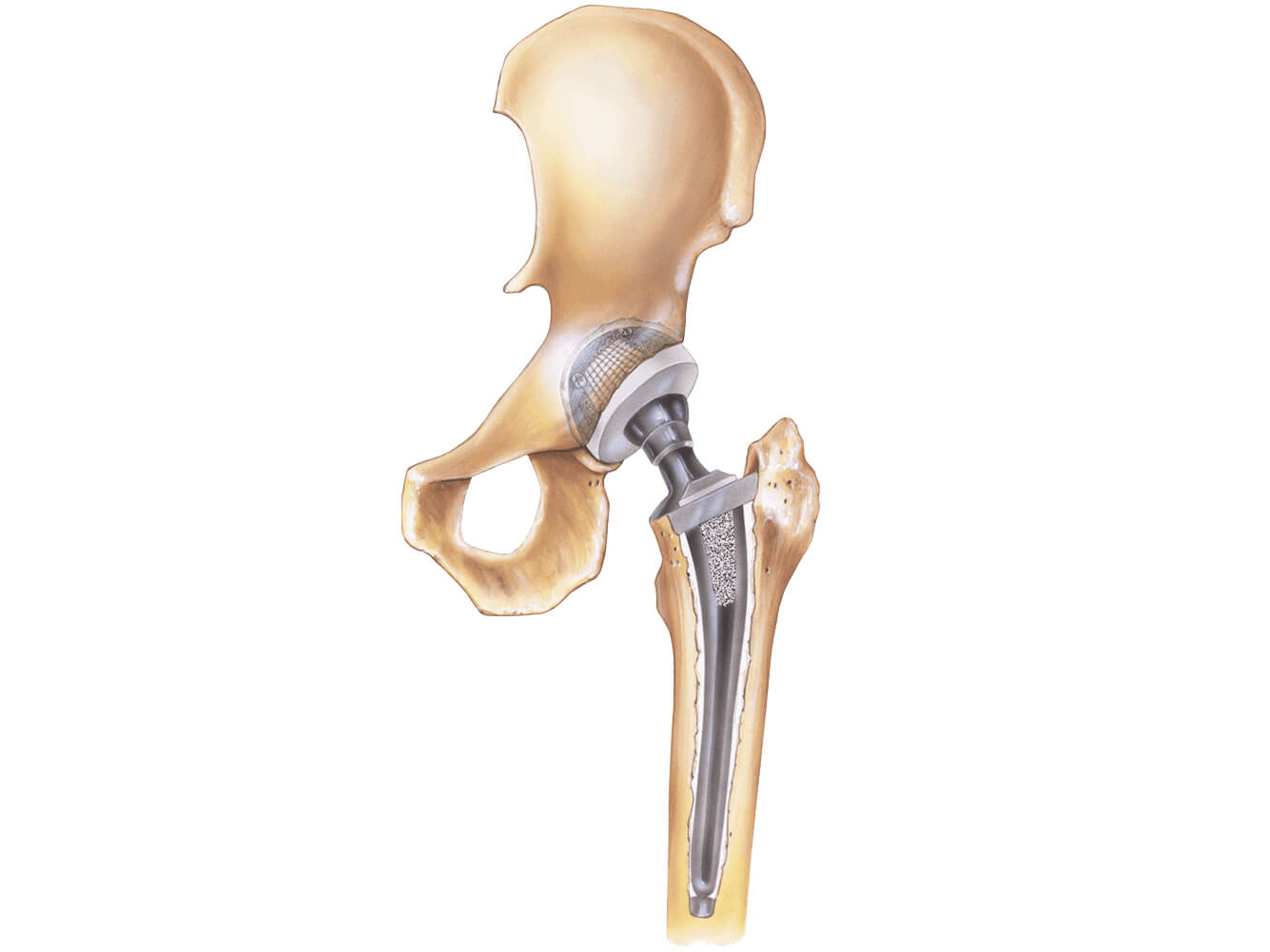
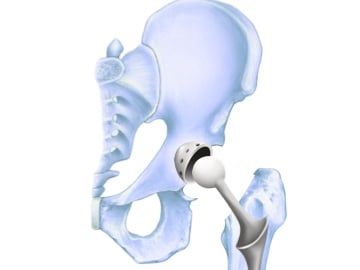
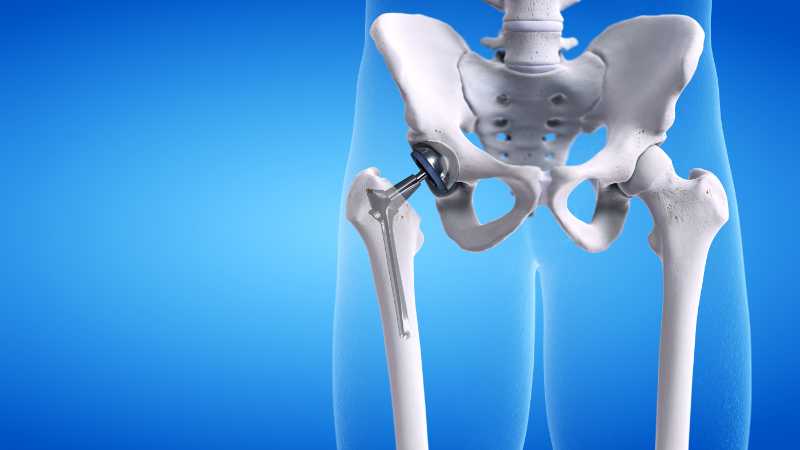
In 1998 depuy and its companies were acquired by johnson johnson and now operate as a subsidiary of the company. Recently on march 8 2013 a los angeles jury awarded loren kransky 8 34 million dollars in the first depuy hip implant trial to go to court. The latter was sold only abroad. Depuy is the maker of several hip systems including the articular surface replacement or asr hip resurfacing system and the asr acetabular hip system both of which were recalled in 2010.
Johnson johnson knee replacement systems have had numerous problems that have resulted in the recall of the product. Depuy s 2010 recall involved the company s articular surface replacement asr acetabular hip system and asr hip resurfacing system. Unfortunately a lot of people have experienced severe complications with their total or partial hip replacement devices. Depuy voluntarily recalled its hip systems after unpublished data from the u k.
In 2017 there were 280 000 hip replacement procedures performed.