Does Salt Make Ice Melt Faster Or Slower

Why does this happen.
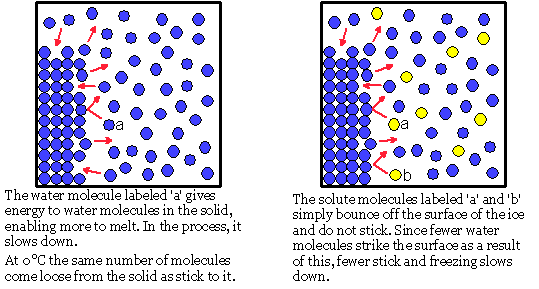
Does salt make ice melt faster or slower. Salt changes the freezing temperature of ice. If it gets colder more water becomes ice. When i put salt on a ice cube it melts the ice very fast and when i saw this i could not believe it. The ice cube in the salt water melts much slower then the one in the freshwater.
Put a teaspoon of table salt on one ice cube. When the ionic compound salt is added to the equation it lowers the freezing point of the water which means the ice on the ground can t freeze that layer of water at 32 f anymore. Put two ice cubes in two glasses. When you add salt it dissolves into the water.
Set the drinking glasses aside somewhere indoors out of direct sunlight. The ice cube without salt melts because the air around it is warmer than 32 degrees f. Boil your water before freezing it to remove air bubbles and make it denser. However if it was ice the salt would melt the ice.
The reason has little if anything to do with convection currents as is demonstrated by the effect of sprinkling dry salt on to the top of an ice cube. One way is to make your own ice cream in a baggie where adding salt to water produces a mixture so cold it can freeze your treat. You can see the ice cube melt away before your eyes much. Thats why they use ice to melt snow off roads.
Salt does not make water freeze slower. In the salt water the colored water from the melted ice cube forms a distinct layer that floats on top of the salt water. If you just want to see an example of how cold ice plus salt can get mix 33 ounces of ordinary table salt with 100. Watch the ice cubes over time.
When you put a freshwater ice cube in a glass of water it melts faster not slower. How do you think the salt sugar and sand will affect how quickly the ice cubes melt. Use salt to melt ice activity you can demonstrate the effect of freezing point depression yourself even if you don t have an icy sidewalk handy. On the other hand if you put the same salt on ice at 15 f the salt will be able to prevent melting ice from re freezing.
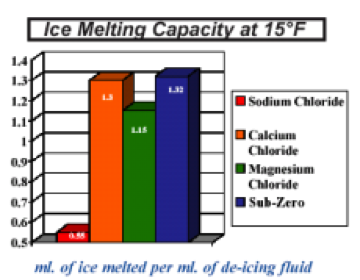
For example tossing table salt sodium chloride onto ice when it s 0 f won t do anything more than coat the ice with a layer of salt. If it gets warmer more ice becomes water. Magnesium chloride works down to 5 f while calcium chloride works down to 20 f. It will freeze and melt more slowly.
The water however can still melt the ice at that temperature which. And if you make your own ice make sure to. Draining the ice as soon as water has accumulated.